Chemical Surface Preparation – The Art of Achieving Fine Lines and Spaces
Michael Carano
RBP Chemical Technology
Article summary
The photoimaging process is one of the first steps in the PCB fabrication process. In order to insure that the image of the circuitry conforms as close to the desired design as possible (i.e. lines and spaces), surface preparation of the copper foil surface is one of the critical success factors. Employing the optimum mix of surface cleaners and microetchants will provide a clean surface with sufficient surface area to promote dry film adhesion. The fabricator has numerous options and should determine the optimum process by accounting for the type of copper foil used as well as the classes of soils to be removed.
Introduction
There are several options available. In addition to pumice and aluminum oxide surface preparation, chemical cleaning as a means to insure optimum photoresist adhesion has gained significant popularity. In this case, only chemical processes such as acid cleaners and micro-etchants are employed. However, chemical cleaning is more than simply utilizing micro-etchants to restructure the copper surface. First the chromate conversion coating must be dealt with.
Chromate Conversion Coating
All copper foil and/or laminate producers process the foil through an anti-tarnish treatment in order to prevent oxidation of the copper surface. This treatment is based on chromic acid. The chromic acid treatment provides a hydrated chromate film on the copper that prevent copper oxidation. While preventing oxidation is necessary during storage, the chromate must be removed prior to micro-etching. Failure to remove the film completely will lead to differential or step-etch during the micro-etching process. The step etch will leave the copper surface with a non-uniform topography. This non uniformity will invariably lead to less than optimum photoresist adhesion. The potential for resist to lock into some of the non uniform areas on the foils is quite high mainly due to the extreme peaks and valleys in the surface profile. The best remedy to prevent this situation is to completely remove the chromate film.
In the past, tarnish resistance was accomplished by immersion of the copper foil into a solution containing chromate ions. Yates and others (1) further improved upon this method with an electrolytic technique to enhance the oxidation resistance of the copper foil. Others later improved upon this invention with the introduction of zinc chromate (2).
One should never underestimate the tenacity of the chromate film. This is precisely why I recommend a strong mineral acid cleaning step prior to pumice, aluminum oxide or chemical microetching. It is much more effective to enhance the resist adhesion when a good chromate removal process is online prior to these additional processes.
Chemical cleaning and micro-etching
First, a review of various chemical cleaning methods is warranted. It is well known that the definition of cleaning is “making the soil soluble in a solvent.” I don’t remember who is responsible for this quote, but it is something I have not forgotten. Basically, one should understand what composition of the soils is and what the proper solvent or solvents are best suited to remove those soils. Chemical compositions designed to remove soils are endless. As an example, Table 1 below provides a succinct summary of those processes. One should also contact the chemical supplier in order to extract advice and counsel on proper operating parameters, equipment compatibility and costs.
Table 1
Chemical Cleaners and Microetchants
| Cleaner class | When to use | Contraindications | comments |
| Alkaline Cleaners | Use with innerlayers prior to oxide or oxide alternative processing. Will also remove light organic soils | Will not remove copper oxides or chromate coatings | Watch for foaming formulations as these may cause issues with cross-contamination |
| Acidic Soak Cleaners | Can be used in spray or immersion mode. If used in spray mode, insure the chemical is low to no foaming | Will not micro-etch. May with the help of certain surfactants remove light organic soils | Combinations of nitric, sulfuric AND hydrochloric acids make excellent chromate removal chemistry. Look at individual acid formulations as well. Phosphoric acid is another good acidic cleaner |
| Microetchants (persulphates, Hydrogen peroxide-sulfuric, Cupric chloride) | Can be used in spray or immersion mode. Control copper removal with proper control of operating parameters | Not designed to remove organic soils | Easy to use. Some etchants can be closed-loop-I.e. recover the etched copper as a copper sulfate salt |
Source: Idea for Table from IPC document 740 (Process Effects)
There are some suppliers that provide one step chromate remover/micro-etchants. Again, one should consult the supplier’s technical data sheet for the proper use and indications. From this writer’s standpoint, the chemical cleaning process is more efficient and effective with at least two separate chemical steps-one as a chromate-soil remover and the second as a copper removal/copper micro-etchant.
Basic Chemical Micro-etching Processes
The basic fundamentals of chemical micro-etchants are quite simple-remove oxides from the surface and restructure the copper foil. The latter means to roughen or create a topography for the copper that enhances photoresist adhesion without excessive copper removal. There are several key points to consider here. First, it is much more effective to create a uniform topography without excessive copper removal if the copper foil surface is already devoid of oils, soils and chromates. Thus the first step in the surface preparation process is to provide virgin surface so that the micro-etch can perform its function. When there are soils and chromates remaining on the surface, the micro-etch will create areas on the surface that for lack of a better term, are referred to as differential or step etch. The topography will exhibit areas of high peaks and low valleys that can promote resist lock-in. Conversely, if there are areas on the foil surface that have deep trenches in the foil due to differential etch, there are concerns with poor resist conformation (Figure 1). In this case, the resist never completely adheres to the copper in these areas. Thus there is a gap that allows for other chemicals to remove copper during the develop-etch-strip process. When other processes are able to remove the copper that was designed to be protected by the resist, the consequence is an open circuit. At the very least one will experience neck downs in the circuit traces.
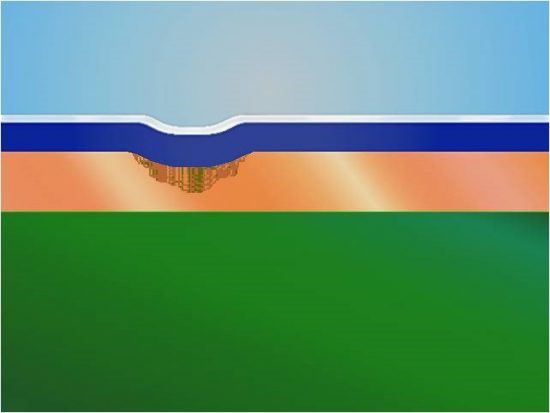
Figure 1-Poor photoresist conformation (source: IPC)
With respect to micro-etchants, there two most commonly used are:
- Persulfate based (sodium or potassium)
- Hydrogen peroxide-sulfuric acid
Persulfate based processes tend to create a much more roughened topography than does hydrogen peroxide-sulfuric acid based processes. However, when the chromate conversion coating is thoroughly removed, both of these etchants are effective.
The angular grain structure promotes sufficient adhesion of the resist to the copper surface. In figure 2, the result of inadequate surface preparation is evident. The exposed resist after developing is lifting from the copper foil surface.

Figure 2-Resist lifting-Source IPC 9121.
Summary
“Making a soil soluble in a solvent.” That is a simple but accurate definition of cleaning. In the case of copper foil surfaces, this suggests that organic soils, chromate anti tarnish coatings and oxides must be removed from the copper prior to micro-etching the foil. The former is accomplished with acid cleaners containing mineral acids, surfactants and other functional materials. Once a clean virgin copper surface is obtained, the fabricator is then able to increase the surface area of the foil with a chemical micro-etch.
References
- U.S. Patent 3,853,716
- U.S. patent 4,387,006



